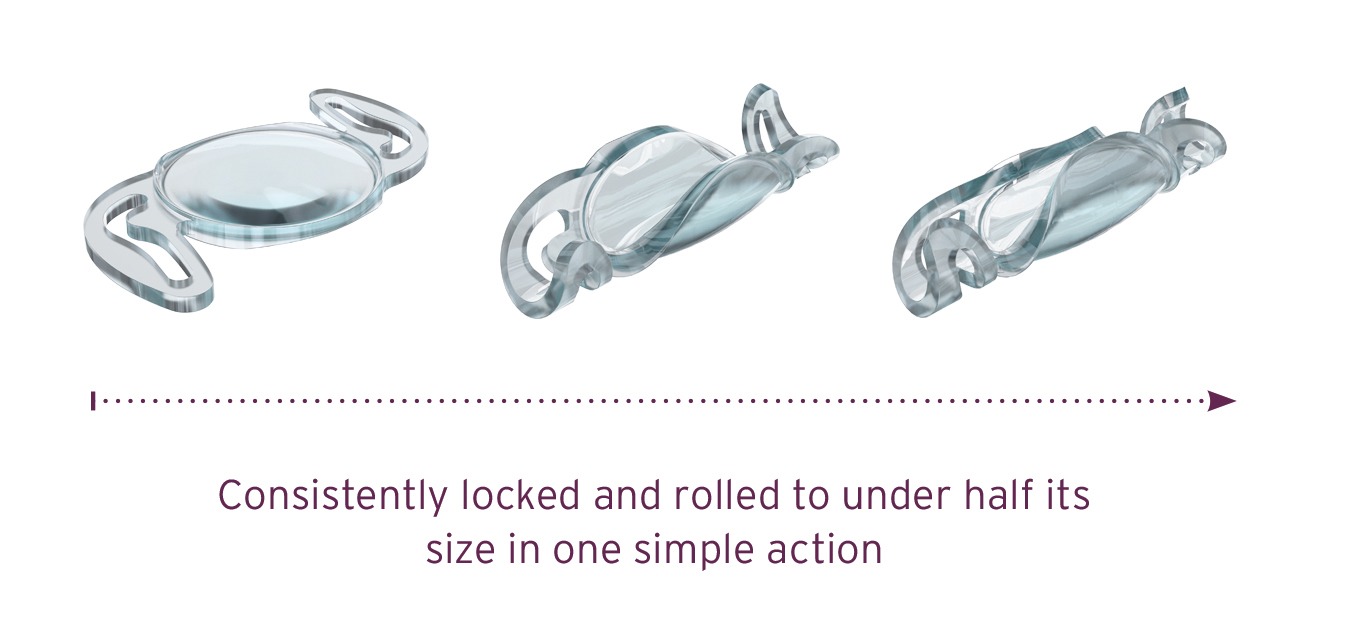
Refractive Cataract Chinese Surgeons Access Rayner IOL
Posted on 27/02/2017
For immediate release. March 02, 2017 – Worthing, United Kingdom. Rayner announced today that a selection of models in their Sulcoflex range of pseudophakic supplementary IOLs have received Chinese Food and Drug Administration (CFDA) approval and are to be launched in market in 2017. Rayner is the first manufacturer to make a supplementary IOL available to surgeons in