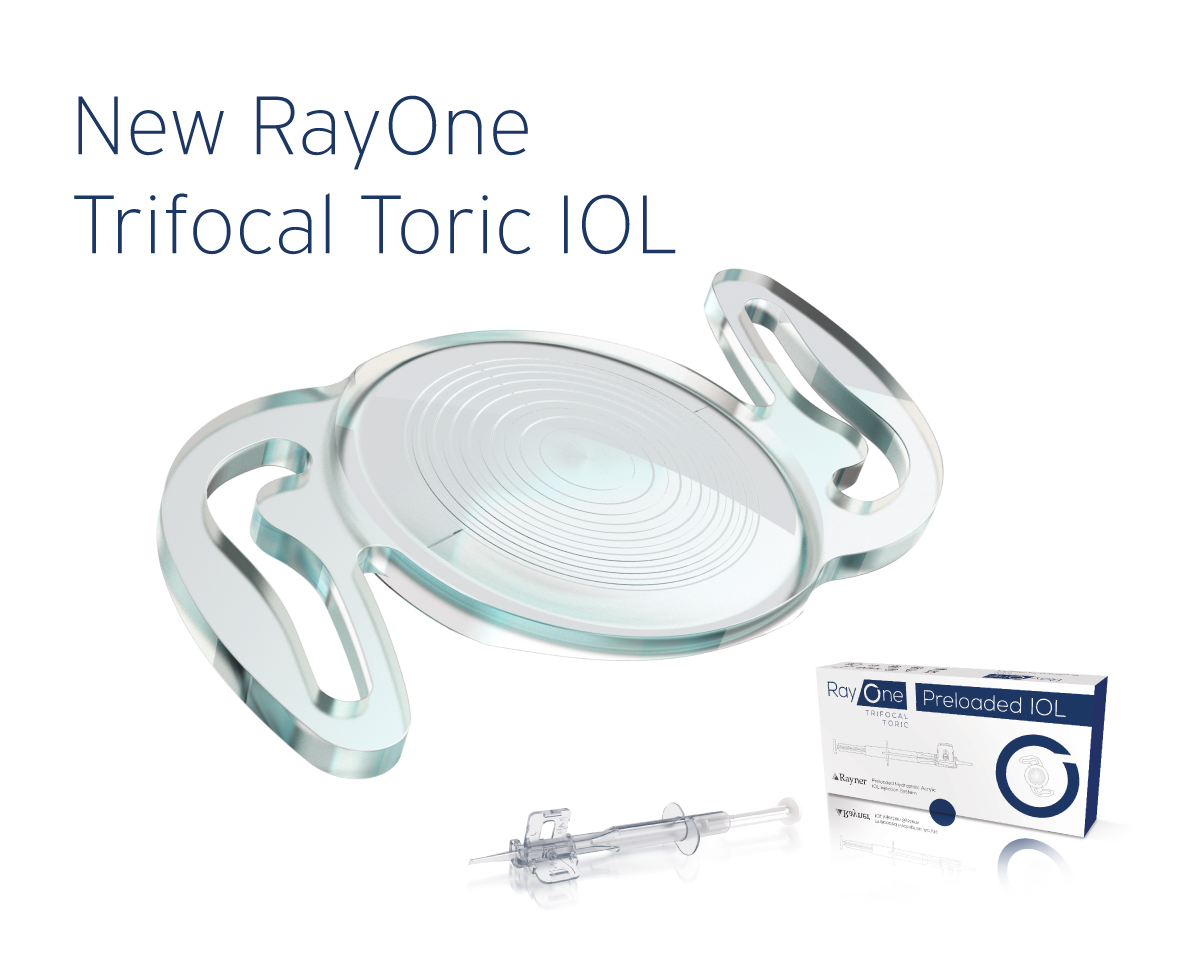
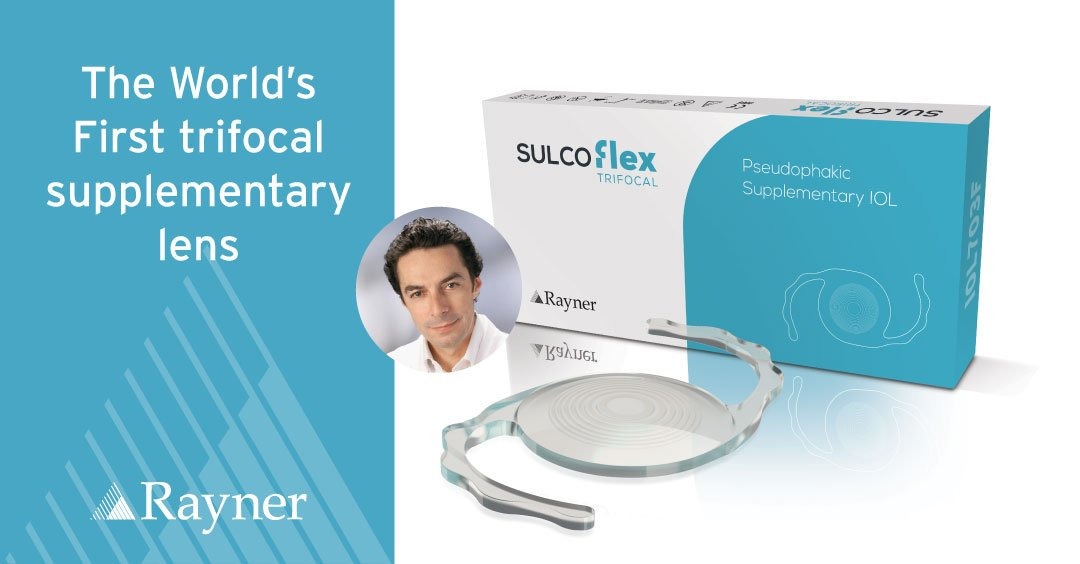
Launching RayOne Trifocal Toric Completes IOL Family
Posted on 11/09/2019
Rayner will offer the most complete family of trifocal IOLs in the industry, allowing surgeons to treat an even wider range of presbyopic patients: Sulcoflex Trifocal, RayOne Trifocal and new RayOne Trifocal Toric. High patient satisfaction and spectacle independence reported from a multicentre pilot study. RayOne Trifocal Toric’s cylinder range starts from +0.75 D, allowing