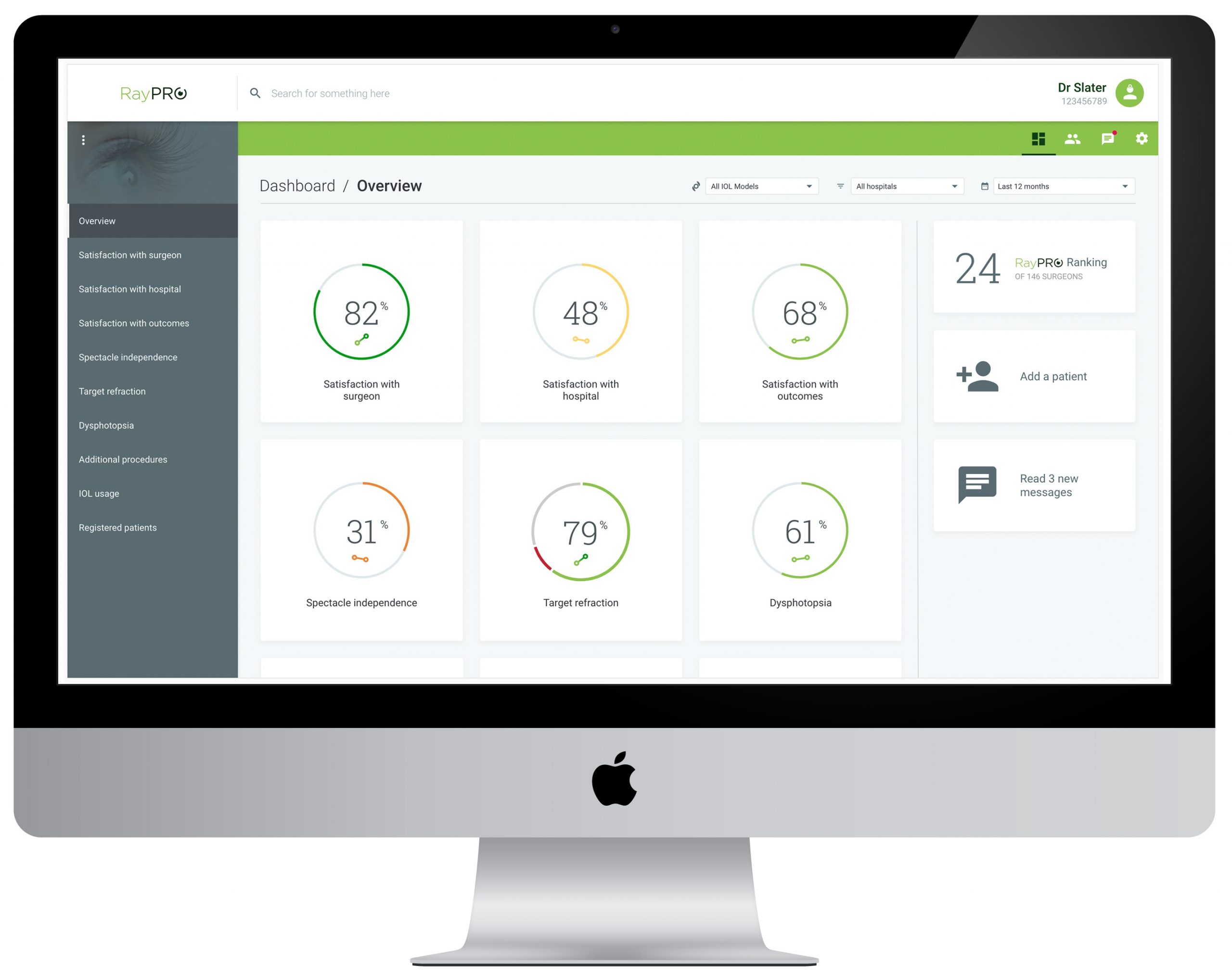
Rayner announces 1,000 IOLs recorded on RayPRO digital platform
Posted on 4/08/2021
AUGUST 04, 2021 – Worthing, United Kingdom. Rayner, the pioneering manufacturer of intraocular lenses for cataract and refractive surgery, announced today that it has achieved the milestone figure of 1,000 intraocular lenses recorded on RayPRO, its digital patient reported outcomes platform. Launched at ASCRS in 2019 for ophthalmic surgeons who perform cataract procedures, RayPRO is